In chemistry class, we learn about a topic called Lewis Dot Structures Diagram where we need to draw dots and bondings between atoms. In this unit, we use Lewis Dot Structures (electron dot diagram) to show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. To draw Lewis Dot Structures, first we need to know the valence electron of the atom (an outer shell electron); the second step will be taking the number of valence electrons and draw it as dots around the atom.
For example, Carbon has 4 valence electrons (an outer shell electron)
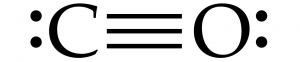
We can also draw bonding between two or more atoms. In a covalent bond, when two electrons bond together, we can draw a line to connect between the two electrons. For example Carbon and Oxygen. The two atoms combine together so that they can share electrons with each other to get eight electrons–which is the number that they want.